They power many different things from razors to radios to remote controls. We all use them, but do we really know what batteries are and how they work?
One common type of battery is the single-use (primary) battery, which is the type you’ll find in most household devices. These batteries contain chemicals which are great for moving electrons, but not so great if they enter a landfill.
Another type of battery is the rechargeable battery. Obviously, these batteries can be recharged and reused multiple times, making them a good option for sustainability. But even rechargeable batteries reach a point where they are no longer useful and need to be recycled.
Let’s look at a typicaldry cell AA battery. On the outside, there is a casing. One end, or terminal, is negative, called an anode. The other end is positive, called the cathode. Both the anode and cathode are also known as electrodes.
The battery’s body separates these electrodes. Within the battery’s body are electrolytes, chemicals which act as a barrier between the anode and cathode.
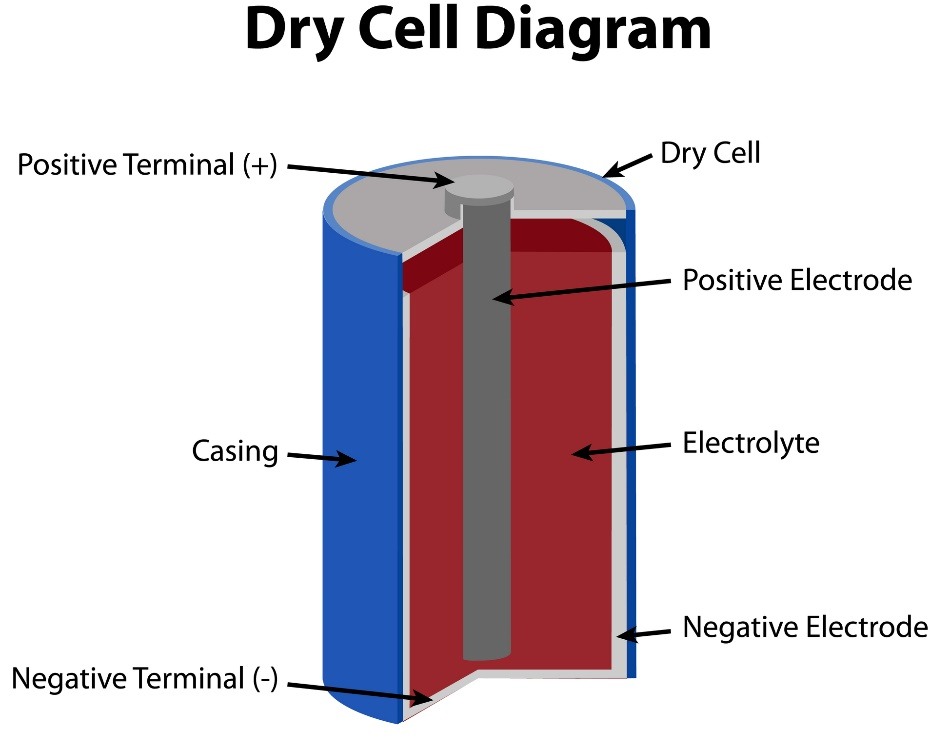
When a battery is not in use, the electrolytes and electrodes are dormant. As soon as you make a circuit, for example, by putting the battery into a flashlight and turning it on, a chemical reaction takes place.
The anode reacts with the electrolytes and produces electrons, which build up at the battery’s negative terminal. The anode is usually made from a material that likes to give up electrons (i.e. zinc).
At the positive terminal, the cathode reacts with electrolytes to create ions — atoms with too few electrons. The cathode is usually made from a metal that likes to collect electrons (i.e. copper).
The electrons want to travel from the anode to the cathode. The electrolytes act as a barrier. When we turn on our flashlight, those electrons find the path of least resistance through the circuit we have created. They flow through the flashlight’s wires and bulb to the cathode, which is how the bulb is illuminated.
A battery can only perform this chemical reaction a certain number of times. After that, the battery can no longer generate a charge, and should be safely recycled.
Fun Experiment
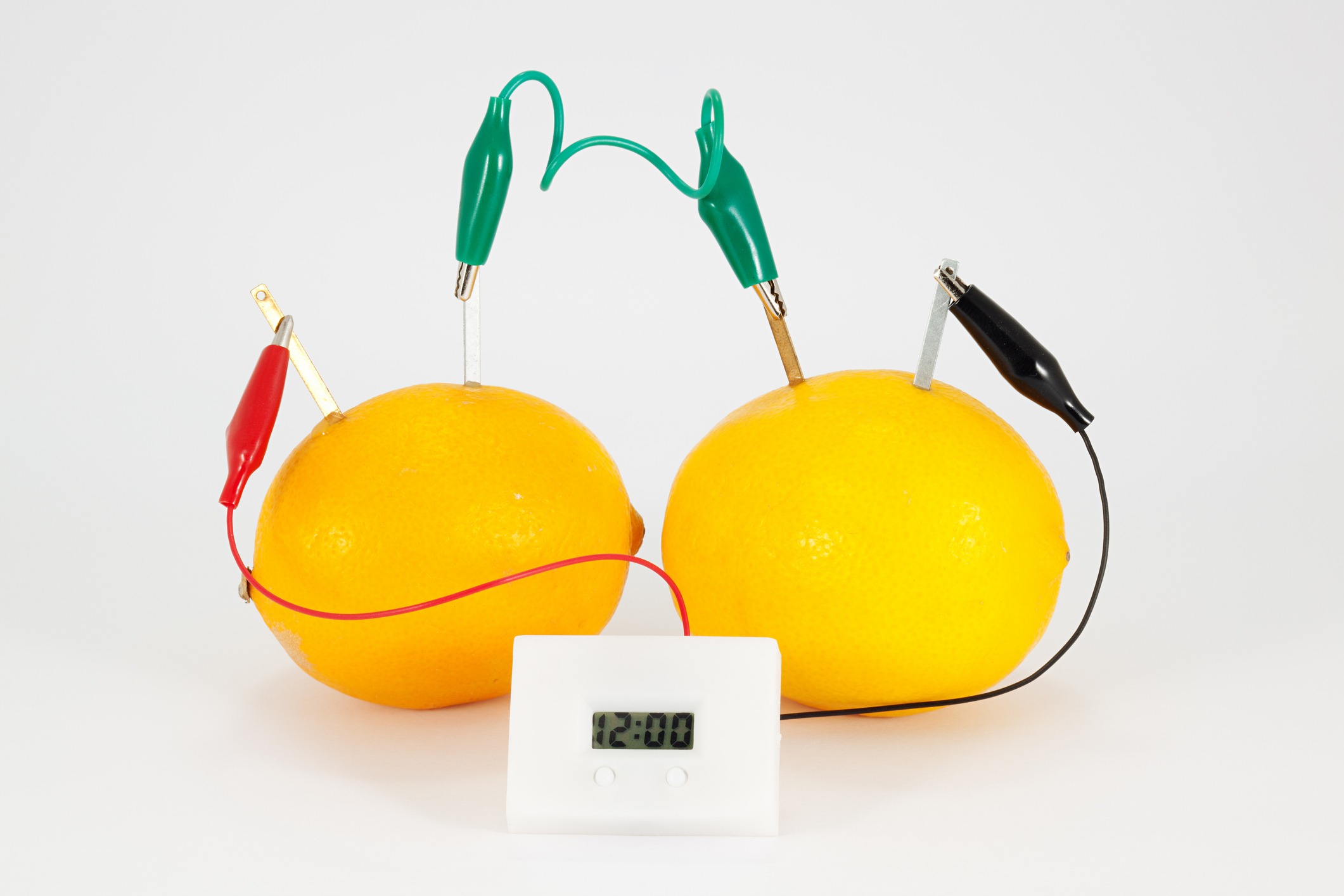
Did you know that you can make a simple battery out of a lemon? All you need is a lemon, some wire, a copper penny, and a zinc nail.
Simply wrap the wire around the penny and the nail and insert each into the lemon, making sure they don’t touch each other.
The acid in the lemon acts as an electrolyte, and the zinc and copper function as the anode and cathode. It won’t be powerful enough to move a car, but you can certainly hook up a small bulb or digital clock to the terminals!
You may also have seen a similar experiment using a potato. The science behind how food can generate electricity is really fascinating. Food contains energy. With the lemon battery we are using zinc and copper electrodes to move that energy.
Electrons flow from one electrode to the other through the acids in the lemon. Just like in a battery you can buy at the store. But don’t try making lemonade out of store-bought batteries!
Batteries may seem like small and insignificant devices, but they play a surprisingly important role in our world. The next time you change a battery, take a moment to appreciate the science inside, and don’t forget to recycle!